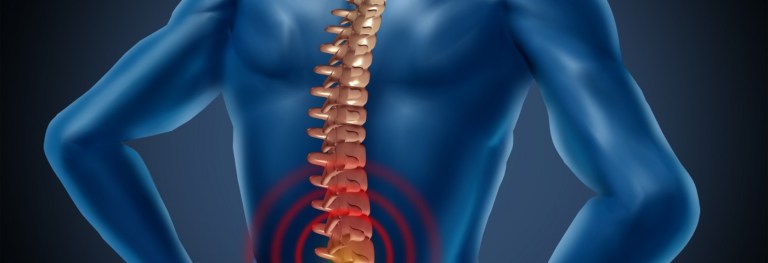
Suffering from fibromyalgia means dealing with pain, sleep disturbances, depression, and thinking and memory problems.
Just as hard can be the skepticism from friends, family and the medical community who doubt the validity of the disease itself.
Little-understood and often misdiagnosed, fibromyalgia affects about 2 percent of the population, according to the Centers for Disease Control and Prevention.
That number is contested by others who say it affects more than 10 million Americans, or an estimated 6 percent of the population, said Dr. Bruce Gillis, founder of Epicgenetics, a biomedical company that developed the FM/a blood test that he says has diagnosed fibromyalgia.
What it is?
Fibromyalgia is a chronic disorder characterized by widespread muscle and joint pain and tenderness either bodywide or in localized areas, along with other symptoms including frequent headaches, poor sleep, restless legs or leg cramps, numbness or tingling.

Click Here to Visit the Store and find Much More….
Among sufferers, it affects about 60 percent women and 40 percent men, Gillis said.
For hundreds of years, fibromyalgia has been misunderstood, Gillis said. Sufferers — mostly women — were labeled neurotic. A century ago women could be institutionalized. In the 1970s, doctors widely prescribed Valium to treat it, Gillis said.
It was classified as a syndrome or a set of symptoms rather than a disease, said Gillis, who previously was in private practice treating patients with fibromyalgia.
To test for fibromyalgia many patients spent three to five years visiting doctors for “rule-out tests,” Gillis said. For example, they were tested and ruled out for lupus or misdiagnosed with rheumatoid arthritis.
With the assistance of major medical universities, including University of California, Los Angeles; University of Illinois at Chicago College of Medicine, and Harvard Medical School, the FM/a test was developed. It diagnoses fibromyalgia by identifying the presence of specific white blood cell abnormalities that have been documented to exist in these patients. The test is FDA-compliant and was introduced in 2013.
The test requires less than a fluid ounce of blood. It analyzes the immune system’s white blood cells’ chemokine and cytokine movement patterns, Gillis said.
Patients with fibromyalgia have an irregular pattern regarding these proteins. Healthcare providers can order the test, which is covered by Medicare and often by insurance.
“I believe we are at the forefront of advancing scientific information about fibromyalgia and answering these critical questions for patients: 1) Do I have fibromyalgia? 2) How and why did I develop fibromyalgia? and 3) Is there a direct treatment for fibromyalgia?” Gillis said.
In an effort to investigate not only the cause of fibromyalgia but to develop an effective treatment, Campaign 250 is looking for 250,000 volunteers to take the FM/a test for free.
Patients who receive a positive diagnosis may elect to participate in a fibromyalgia-specific vaccine clinical trial that will seek to alleviate fibromyalgia-related symptoms.
The study will be led by Dr. Denise Faustman, director of Immunobiology Laboratories, Massachusetts General Hospital/Harvard School of Medicine, who is studying common markets and DNA traits in her research in an FM vaccine.
“No longer do people with fibromyalgia have to listen to others say ‘It’s all in your head’ or ‘She looks perfectly fine.’ You can’t see the immune system at work,” Gillis said.
Source>http://www.monroenews.com/news/20170711/new-test-can-detect-fibromyalgia
[mailpoet_form id=”2″]

Why the baseball reference in the middle of this article???
Where is it?
About paragraph 7 in the What is it section. Here is the paragraph:
Monroe’s Owen Yount singles home the go-ahead run in the fourth inning against Frenchtown at the Monroe County Fair Tournament Sunday.
It diagnoses fibromyalgia by identifying the presence of specific white blood cell abnormalities that have been documented to exist in these patients. The test is FDA-compliant and was introduced in 2013.
Removed. Sorry for error.